Series
CHEMISTRY 1110 PRACTICE EXAMS (Order of topics and class specific info below could be different than current) Study for exam and then take practice exam without key. Grade yourself with key to see what you know. Use results to guide additional study and then repeat with another practice exam. 1110Exam 1 Fall 2007 1110Exam 1 Fall 2007 KEY. Unit 1 - Investigating Chemical Reactions Study Guide Page 1 Chemistry 1102 To fulfill the objectives of this unit, students should complete the following: Reading for this unit: Science 10 Chapter 5: Introduction: pages 170-171 Section 5.1: pages 172-174 Investigation 5.3: pages 180-182 Handout 1: “WHMIS Activity”: Appendix A. Chemical Equations and Reactions MIXED REVIEW SHORT ANSWER Answer the following questions in the space provided. B A balanced chemical equation represents all the following except (a) experimentally established facts. (b) the mechanism by which reactants combine to form products. (c) identities of reactants and products in a chemical reaction.
A video instructional series in basic chemistry concepts and science history.
Chemistry Chemical Reactions Study Guide Key Answers
A video-based instructional series in chemistry with accompanying website for high school and college classes. 13 half-hour programs, online text, course guide, interactive lessons, historical timeline and periodic table.
Chemistry: Challenges and Solutions teaches general chemistry concepts using real-life challenges in energy, materials development, biochemistry, and the environment.
The course zeroes in on essential topics that are generally taught in introductory chemistry, providing a strong foundation for learners to pursue further study in science or a liberal-arts education. Videos include dramatic demonstrations of key principles, interviews with scientists who are doing current research related to these fields, animations, and clear explanations. Each video is hosted by a different working chemist – together, they show a diversity of chemistry professionals and the challenges chemistry is addressing for society. The on-line text covers key concepts with clear text and illustrations, while interactive labs provide simulations of chemical processes online.
Course Goals
Energy resources, materials science, healthcare, and the need to understand new developments in chemistry and biotechnology are challenging today’s learners to become more literate in how chemistry affects our lives every day. Chemistry: Challenges and Solutions is designed to cover basic chemistry concepts appropriate for an introductory, qualitative approach to the subject, and to do so in a way that emphasizes the key importance of chemistry to our daily lives.
With human welfare front and center, one of the primary goals of the course is to help learners fully participate in the public discourse about current challenges facing society. This is a discourse that will require a basic grasp of the underlying principles governing the interactions of matter and energy, all of which are at the heart of the study of chemistry. Learners who successfully complete the course should have sufficient understanding of the atomic model of matter and be able to apply this model to a variety of technical and scientific contexts. In addition, the course is designed to assist learners who are on a pathway to careers in STEM (science, technology, mathematics, and engineering) where further learning in the health sciences, environmental science, biotechnology, and other fields requires an understanding of basic chemistry. For these learners, the practical application of chemistry to their chosen fields is evident through the many virtual site visits to laboratories, research centers, manufacturing sites, and other facilities featured in the video programs. Finally, for those learners who are motivated by curiosity or who desire to round out their knowledge of a terrain which may be unfamiliar or perhaps even perceived as unfriendly, we embrace you: One of the course’s primary goals is to instill a love of the subject in its elegance and simplicity.
To achieve these learning goals, the course is built around the following objectives:
- To teach basic chemistry principles using only basic mathematics through text, illustrations, animations, and video. The multimedia format of the course allows learners many modalities and entry points to topics.
- To motivate students to learn chemistry by demonstrating its relevance to real-world problems, showing chemists as role models in improving human lives. Video profiles of science research teams, as well as numerous sidebars and historical examples in the text, further the objective of humanizing science.
- To teach about the nature of science by linking key modern concepts to the historical development of chemical ideas. Past discoveries are placed in context of continually evolving models, which though incomplete or incorrect by today’s standards, serve to advance the field in spite of their limitations.
Chemistry is a large field with many branches and subspecialties. To remain manageable in scope, Chemistry: Challenges and Solutions focuses on essential topics that are generally taught in introductory chemistry, providing a strong foundation for learners to pursue future study in science or a liberal arts education. The focus in the course is to keep returning to key, fundamental ideas, such as the particulate nature of matter, the conservation of matter and energy, and basic quantum mechanical principles, which learners will apply again and again in different situations. For those who wish to explore more broadly, suggestions for further reading are included at the end of each unit. The Course Guide is an additional resource for classroom activities and learning resources.
Chemistry: Challenges and Solutions is designed to be adaptable to a variety of settings: classrooms, STEM programs, higher education distance learning, individual or instructor-moderated online learning, and professional development. Whatever the purpose, navigation is provided so that the materials can be used sequentially or in any order. Navigation and search tools provide easy access to content either by individual learners or by facilitators using the materials as a stand-alone course or as a way to extend and augment their existing course offerings.
About Demo Dan
Videos are enriched with chemistry demonstrations by Dan Rosenberg of the Harvard University Natural Sciences Lecture Demonstrations Department. “Demo Dan” doesn’t just make things go “Boom!”—his segments clearly connect key chemical concepts. Dan not only does things you don’t want to do at home but makes chemistry come alive for beginning students.
Course Audience
The target audience for the course is primarily introductory learners. These include junior and senior level high school students to first and second year college students. It will be appropriate for majors and non-science majors, and can serve a variety of audiences, including:
- Supplementary learning for chemistry classes. Videos (especially the demos), interactive labs, and course readings are a rich resource to reinforce key concepts.
- Out-of-school STEM programs. These include after-school and Saturday enrichment academies for learners with particular interest in chemistry or STEM in general.
- Higher education distance learning.
- Specialized technical programs such as nursing, where chemistry is a required course.
- Interested adult learners who want to learn more about the subject on their own.
- High school faculty seeking professional development.
For teachers, the Course Guide has supplemental readings and discussion/reflection topics designed to help educators thoughtfully address student misconceptions and different learning styles in their classrooms. The course can also enhance teaching and learning by showing current, real-world examples of chemistry applications as motivators for learning.
Course Components
At the center of the course is a website that provides access to all its components. On the site, there are thirteen separate units, whose sequence roughly follows the historical progression of the field from prehistoric cave paintings to modern materials. The website provides links to key multimedia materials, which include:
- Thirteen half-hour videos: Broadcast-quality videos are hosted by chemists of different backgrounds. Each video focuses on a few key topics and illustrates them using the host, demonstrations, and visits to research laboratories or other sites where chemistry is put into practice.
- Three interactive components: the Chemistry Timeline, which provides a visual display of key chemists and their discoveries, as well as two virtual labs: Control a Haber-Bosch Ammonia Plant and The Chemistry of Running. The interactive labs provide a challenge (maximize profit from ammonia synthesis or successfully complete a marathon) that reinforces the practical applications of chemistry concepts covered in the units.
- Visuals: A compilation of images and animations linked to each unit with captions. These allow easy access to visual materials in any order.
- Course Guide: Designed for instructors and course facilitators, the course guide provides resources that take facilitators step-by-step through each unit. The Course Guide is available as a downloadable PDF.
- Glossary: New vocabulary terms in the text are highlighted in bold, and defined in the Glossary.
- The Periodic Table of the Elements is an additional interactive that has information and background on all of the known elements, linked to relevant sections in the text.
Taken together, the course has over six hours of video and 300 pages of web text, which can be integrated into a progressive learning experience or indexed and organized for easy access to any section.
How to Use This Course
The scope and sequence closely follow the topics presented in many introductory chemistry courses, but with a strong emphasis on historical connections as well as real-life applications. The course primarily follows a traditional, step-by-step approach. Mac os el capitan iso image download. It is loosely based on the historical development of chemistry ideas in a sequence that would be applicable to a one-semester introductory chemistry course, with materials designed for maximum flexibility for different users. For instructors interested in using the materials as the basis for a traditional one-semester course, each of the thirteen units could be covered in approximately one week. That includes class time for demonstrations, readings, viewing the videos, and introducing problem sets provided by the instructor. For individual learners or small groups, the videos should be watched first, and if used in a group setting, preferably during meeting time to provide a shared experience for the suggested discussion and reflection questions. For instructors interested in using the course as part of a “flipped classroom” model, the videos are sufficiently clear and self-contained that they could be viewed by the students at home, leaving class time available for individual instruction, project-based learning, or laboratory activities.
For introductory students, it is advisable to start at the beginning, as key concepts are introduced in a logical order that generally follows a historical progression of the development of chemical ideas. Of course, some learners may want to explore a single section of the materials for self-directed learning about a particular topic. For these learners, the search tools and the navigation of the website can help them quickly locate sections of interest. Since every component of the course is also designed to stand alone, learners do not need to use all of the materials or access them in any particular order. If interested in a particular topic, users can jump in at a point of interest using the search or the many navigation guides. For the fullest experience with all the components, go to the Course Guide to see the learning goals and to explore the demonstrations, discussion questions, and additional resources available for many of the key course concepts. Information on how to use course materials to facilitate a professional development workshop is also contained in the Course Guide.
Although the videos are 30 minutes long, these, too, are accessible to be watched in sections or in other non-traditional ways. Each video is broken into five-to-eight discrete segments, identified by on-screen titles. Users can find sections by scrolling forward or back until they see the title.
Course Content Credits
Lead Content Developer:
Dr. Christopher Morse, Lecturer in Chemistry, Olin College.
Dr. Morse earned his doctoral degree in inorganic chemistry at the Massachusetts Institute of Technology. Before coming to Olin College, he was a faculty member in the Chemistry Department at Tufts University. At Olin, Dr. Morse teaches courses in general and organic chemistry and has co-authored a textbook for a course about art, art history, and art preservation from a chemical perspective. El capitan image download. Additionally, Dr. Morse is the science editor at Sporcle.com, a quiz site where he coordinates and creates study guides and chemistry quizzes for students.
Writers:
Writers were chosen to represent the field’s different backgrounds and areas of expertise, as well as for the breadth of their knowledge and their experience communicating the topic to students and non-scientists.
- Asst. Prof. Karen Atkinson, Bunker Hill Community College
- Asst. Prof. Adam Brunet, American International College
- Louisa Morrison, Instructor, Wellesley High School, Wellesley, MA
- Thomas van Geel, Instructor, Wellesley High School, Wellesley, MA
- Jennifer Weeks, Science Journalist
Advisors:
Advisors were selected to represent the broadest variety of perspectives in chemistry research, education, outreach, and communication. Advisors met first to outline the parameters for the course, and again when the materials were available, to review and provide mid-course corrections. See the production credits for the complete list of advisors.
On-Camera Hosts:
On-camera hosts were carefully selected to represent a variety of approaches within the field, as well as to challenge stereotypes about chemists. One thing they all have in common is the ability to explain clearly and concisely in an engaging way. See the production credits for the complete list of on-camera hosts.
Production Team:
Harvard-Smithsonian Center for Astrophysics
Course materials were developed at the Science Media Group, the media production unit of the Science Education Department at the Smithsonian Astrophysical Observatory, a member of the Harvard-Smithsonian Center for Astrophysics. The Science Media Group is the same team that has produced other STEM-based courses for Learner.org, including The Habitable Planet: A Systems Approach to Environmental Science, Physics for the 21st Century, and Neuroscience & the Classroom: Making Connections. Executive Director: Dr. Matthew H. Schneps.
Production Credits
The Science Media Group (SMG) was founded by Dr. Matthew H. Schneps at the Harvard-Smithsonian Center for Astrophysics (CfA) to investigate new ways to use television and computer media to communicate science to the public at large. A part of the CfA’s Science Education Department, the SMG has grounded its work in state-of-the-art science education research.
The most recent SMG production was Neuroscience and the Classroom: Making Connections—a course to help teachers learn to use research to create their own solutions to their particular classroom challenges. Other recent SMG productions were Physics for the 21st Century, a course for high school physics teachers and undergraduate students exploring the frontiers of physics; and The Habitable Planet: A Systems Approach to Environmental Science, a course for high school teachers and undergraduate students in environmental science. Created in partnership with the Harvard University Center for the Environment and Annenberg Learner (then Annenberg Media), The Habitable Planet received the 2010 AAAS Science Prize for Online Resources in Education (SPORE). Other pioneering works created by the SMG are videos, such as A Private Universe, influential in shaping education reform worldwide; television programs, such as the PBS series Minds of Our Own, designed to alert the public to issues of science learning; and television, museum, Internet, and computer materials for children, parents, and teachers.
Project Development
- Executive Director
- Matthew H. Schneps
- Executive Producer
- Alex Griswold
- Education Coordinator
- Nancy Finkelstein
- Course Developer
- Christopher Morse
- Project Administrator
- Linda P. Williamson
Series Credits
- Project Advisors
- Karen Atkinson
- Chris Bauer
- Adam Brunet
- Noah Finkelstein
- Myriam Hibbard
- Mary Kirchhoff
- Paul Konowicz
- Laurie Langdon
- Robert Miller
- Louisa Morrison
- Daniel Rosenberg
- Wolfgang Rueckner
- Kimberly Stieglitz
- Writers
- Karen Atkinson
- Adam Brunet
- Louisa Morrison
- Christopher Morse
- Thomas van Geel
- Jenny Weeks
- Copy Editor
- Linda Walsh
- Reading Level Specialist
- Ellen Lewis
- Course Evaluation
- Lisa Allen
- Bill Bowers
- Mary Christian-Madden
- Chris Koutros
- Mia Mitoma
- Dick Moran
- Lois K. Ongley
- William Palmer
- MaryJac Reed
- Kathy Siok
- Ruth Tanner
- Martin Isaks
Website Create el capitan install usb.
- Designer
- Shira Fruchtman
- Developers
- Shira Fruchtman
- Michelle Hardy Kennedy
- Programmers
- Aladdin Ibrahim
- Michelle Hardy Kennedy
- Production Assistants
- Diana Eastman
- Grace Hewett
- Ruthann Hewett
Interactive Labs
- Designers
- Shira Fruchtman
- Michelle Hardy Kennedy
- Programmer
- Aladdin Ibrahim
- Interactive Researcher
- Alex Griswold
- Writer/Coordinator
- Susan Sunbury
- Production Assistant
- Erin Braswell
- Interactive Advisors
- Carol Lund
- Peter G. Weyand
Course Guide
- Writers
- Karen Atkinson
- Louisa Morrison
- Thomas van Geel
- Teacher Review
- Nancy Finkelstein
- Copy Editor
- Nancy Finkelstein
- Editors
- Lauren Farrer
- Nancy Finkelstein
Video Production
- Senior Producer | Videographer
- Clive Grainger
- Producer | Videographer
- Tobias McElheny
- Senior Editor | Producer
- Steven J. Allardi
- Editors | Producers
- L. Neal Duffy
- Keri Green
- Associate Producers | Videographers
- Lauren Farrer
- Molly Wasser
- Editor
- Oren Bendavid-Val
- Online Editor
- Maria Kobrina
- Animator
- Rachel D’Erminio
- Production Assistant | Videographer
- Teresa Hartmann
- Music
- Ben Cosgrove
- Alison Plante—Treble Cove Music
- Narrator
- Anna Lewicke
- Hosts
- Adam Brunet
- Catherine L. Drennan
- Nicole Labbe
- Michael McCarthy
- Christopher Morse
- Mala Radhakrishnan
- Ainissa Ramirez
- David Song
- Elizabeth Vogel Taylor
- Wilton L. Virgo
Contributor Biographies
Writers
Karen Atkinson
Dr. Karen Atkinson is an associate professor of chemistry at Bunker Hill Community College (BHCC). Dr. Atkinson earned her B.A. in chemistry and medieval/renaissance studies from Wellesley College and her doctoral degree in chemistry from Northeastern University. She has prior teaching experience at Northeastern’s University College (now the College of Professional Studies) and Boston College. Dr. Atkinson serves as the liaison for BHCC’s Science and Engineering Department for various outside research opportunities for BHCC students, including at Boston University, Massachusetts Institute of Technology, and Wellesley College. She also developed (and teaches) the department’s science writing elective course.
Adam Brunet
Dr. Adam Brunet is an assistant professor of chemistry at American International College. Dr. Brunet obtained degrees in biology and biochemistry from American International College, a private, liberal arts college in Springfield, Massachusetts, then went on to Princeton University for his doctoral degree in chemistry and to the University of Massachusetts for a master’s degree in business administration. He returned to American International College in 2008 to teach and currently teaches organic chemistry and biochemistry.
Louisa Morrison
Louisa Morrison is a chemistry teacher at Wellesley High School in Massachusetts. Ms. Morrison studied chemistry at Barnard College and obtained her M.S. in biochemistry and toxicology from Vanderbilt University Medical Center and an M.A. in Teaching from Belmont University. She worked in biochemical research at Woods Hole Oceanographic Institution and Vanderbilt University. She began teaching high school chemistry in the Nashville Public Schools from 2009 to 2011 and is currently teaching at Wellesley High School, a public school in Wellesley, Massachusetts.
Christopher Morse
Dr. Christopher Morse is a Lecturer in Chemistry at Olin College. Dr. Morse studied at Dartmouth College before earning his doctoral degree in inorganic chemistry at the Massachusetts Institute of Technology in the lab of Alan Davison where he was a National Science Foundation Predoctoral Fellow. Before coming to Olin College, Dr. Morse was a faculty member in the chemistry department at Tufts University where his courses covered both graduate and undergraduate curricula. At Tufts, he successfully ran the Summer Institute on College Teaching for seven years and served as the Graduate Training Coordinator, with the responsibility for the pedagogical training of the graduate students, especially those interested in careers in academia. At Olin, Dr. Morse teaches courses in general and organic chemistry. He recently co-authored a textbook for a course about art, art history and art preservation from a chemical perspective. Additionally, Dr. Morse is the science editor at Sporcle.com, a quiz site where he coordinates and creates study guides and chemistry quizzes for students.
Thomas van Geel
Dr. Thomas van Geel has taught chemistry at Wellesley (Massachusetts) High School since 2005. Dr. van Geel holds an M.D., and prior to coming to WHS, he was a teaching fellow at Harvard University.
Jennifer Weeks
Jennifer Weeks worked for fifteen years as a Congressional staffer and public policy analyst before becoming a freelance science writer. She graduated from Williams College and earned master’s degrees at the University of North Carolina (political science) and Harvard University (environmental policy). As a freelance journalist, Ms. Weeks has written for more than 50 newspapers, magazines, and web sites, and worked on diverse projects for nonprofit and government clients. She is a contributing writer for CQ Researcher magazine, published by Congressional Quarterly Press, and belongs to the Society of Environmental Journalists and the National Association of Science Writers. She has covered topics including energy, climate change, organic food, agriculture, land and wildlife conservation, waste management, and earth science, in formats ranging from news reports to service articles, detailed features, and book reviews.
Video Hosts
Adam Brunet
See Adam Brunet’s biography above.
Catherine L. Drennan
Dr. Catherine L. Drennan is a professor of chemistry and biology at the Massachusetts Institute of Technology and a professor and investigator with the Howard Hughes Medical Institute. Dedicated to both research and teaching, Dr. Drennan’s educational initiatives include creating free resources for educators that help students recognize the underlying chemical principles in biology and medicine, and that train graduate student teaching assistants and mentors to be effective teacher-scholars. Dr. Drennan earned her doctoral degree from the University of Michigan and her primary research interest is the use of X-ray crystallography to study the structure and mechanism of metalloproteins.
Nicole Labbe
Dr. Nicole Labbe is a postdoctoral researcher in chemical sciences and engineering at Argonne National Laboratory working on electronic structure theory and kinetic modeling for biofuel combustion applications. In the Fall of 2012, she earned her Ph.D. in chemical engineering at the University of Massachusetts Amherst where she was a National Defense Science and Engineering Graduate Fellow.
Michael McCarthy
Dr. Michael C. McCarthy is a staff scientist at the Harvard-Smithsonian Center for Astrophysics in Cambridge, Massachusetts and an associate of the School of Engineering and Applied Sciences at Harvard University. He earned his B.S. from the University of Alaska and his Ph.D. in physical chemistry from the Massachusetts Institute of Technology. Affiliated with the Center for Astrophysics’ Spectroscopy Group, his research centers around the production and identification of molecules in the interstellar medium; microwave and laser spectroscopy of carbon chains, carbon rings, and carbon clusters; and chemistry of the interstellar gas and molecular radio astronomy.
Chemistry Chapter 10 Chemical Reactions Study Guide Answers
Christopher Morse
See Chris Morse’s biography above.
Mala Radhakrishnan
Dr. Mala Radhakrishnan is an assistant professor in the Chemistry Department at Wellesley College. She earned her A.B. in chemistry and physics from Harvard College and her Ph.D. in physical chemistry from the Massachusetts Institute of Technology. Her research involves analyzing, developing, and applying computational methods to design and study drugs and other biologically-relevant molecules and systems. She is passionate about interdisciplinary and collaborative research and the intersection of science and creativity. To that end, she is the author of a humorous collection of educational chemistry poetry entitled: “Atomic Romances, Molecular Dances” and spent many years performing her chemistry poetry to diverse audiences in the Boston area. She is an avid musician and enjoys spending time with her family.
Ainissa Ramirez
Dr. Ainissa Ramirez is a materials scientist turned science evangelist, dedicated to sharing the joy of materials, science, and creativity with students of all ages. After undergraduate studies at Brown University, she went on to obtain her Ph.D. in materials science and engineering at Stanford University. She joined the faculty at Yale University in 2003, and was an associate professor of mechanical engineering and materials science for 10 years, leading a research program in smart materials and nanomaterials when she took on the call to improve science understanding. Prior to Yale, Dr. Ramirez worked at Bell Laboratories as a member of technical staff, where she developed a universal solder (a reactive metal that bonds to glass) for which she was awarded MIT’s Technology Review TR100 award. She has published 50 scientific articles and has been featured in The New York Times, CNN, ESPN, and Forbes. Her outreach, including two video series—Material Marvels (www.materialmarvels.com) and Science Xplained (www.sciencexplained.com)—has been widely disseminated on YouTube and promoted by Scientific American.
David Song
David Song is a graduate student and National Science Foundation Pre-doctoral Fellow in the Nocera laboratory at Harvard University. The Nocera Group studies the basic mechanisms of energy conversion in biology and chemistry with the aim of delivering a carbon-neutral and sustainable energy supply for the 21st century. Collaborating with Prof. JoAnne Stubbe at MIT, Mr. Song’s research focuses on understanding how biological molecules, such as enzymes, control the bioenergetics of metabolism through a reaction called proton-coupled electron transfer (PCET). Mr. Song graduated from the University of Illinois at Urbana-Champaign with a degree in chemistry and molecular and cellular biology in 2010.
Elizabeth Vogel Taylor
Dr. Elizabeth Vogel Taylor is a Howard Hughes Medical Institute Instructor in the Chemistry Department at the Massachusetts Institute of Technology (MIT). Dr. Taylor’s educational interests focus on generating free and easy-to-implement materials for chemistry and biology educators. Projects include creating and assessing examples for general chemistry that demonstrate the chemical principles behind applications in biology, medicine, and MIT research and a series of two-minute videos that illuminate the who and the why of chemistry. Dr. Taylor is also involved in graduate student teaching assistant and mentor training and has taught biochemistry laboratory modules and freshman chemistry. Dr. Taylor received her Ph.D. in organic chemistry from MIT in 2006.
Wilton L. Virgo
Dr. Wilton L. Virgo is a quantum physical chemist with more than 10 years of expertise in performing state-of-the-art research in laser spectroscopy and the development of molecular theory. Dr. Virgo’s cutting-edge work has been published in the top scientific physics and chemistry journals. He has authored two books, “Quantum Mechanics in Everyday Life” and “The Equations of Chemistry,” for the advancement of science education. Dr. Virgo received his Ph.D. from Arizona State University and has performed chemical physics laser research at Princeton University, Brookhaven National Laboratory, Arizona State University, MIT and Wellesley College.
Project Advisors
Karen Atkinson
See Karen Atkinson’s biography above.
Christopher Bauer
Dr. Christopher F. Bauer is a Professor of Chemistry at the University of New Hampshire. After earning his Ph.D. at Colorado State University and starting his academic career in environmental analytical chemistry, he redirected his attention to chemistry teaching and learning. His research interests address college-level science instruction student misconceptions, student attitudes and motivation, discovery-based curricula, and faculty beliefs and practices. He has designed and presented many workshops on teaching and learning for elementary, secondary, and university teachers. He was recognized for outstanding teaching with the 1992 University of New Hampshire’s Jean Brierley teaching award and recently held the first Ted Ashford Fellowship from the ACS Examinations Institute. He has working relationships with the major curriculum projects Peer-Led Team Learning, Calibrated Peer Review, and POGIL. He participates in several ongoing NSF projects as an evaluation consultant. At UNH, his primary teaching responsibilities include General Chemistry for biological and physical sciences, Peer-led Team Learning, and a graduate course in Teaching and Learning in Science for STEM graduate students. He was chair of the 2007 Gordon Conference on Chemical Education Research and Practice.
Adam Brunet
See Adam Brunet’s biography above.
Noah Finkelstein
Dr. Noah Finkelstein is a Professor of Physics at the University of Colorado (CU) Boulder and conducts research in physics education. He serves as a director of both the Physics Education Research (PER) group at Colorado, and an NSF-funded Center for STEM Learning, recently launched at CU. Dr. Finkelstein’s research focuses on studying the conditions that support students’ interest and ability in physics—developing models of context. These research projects range from the specifics of student learning particular concepts, to the departmental and institutional scales of sustainable educational transformation. This research has resulted in nearly 100 publications. He is increasingly involved in education policy, serving on many national boards including chairing the American Physical Society’s Committee on Education, and is a Technical Advisor to the Association of American University’s STEM Education Initiative. He is a Fellow of the American Physical Society and a Presidential Teaching Scholar for the University of Colorado system. Dr. Finkelstein earned his Ph.D. in applied physics from Princeton University.
Myriam Hibbard
Myriam Hibbard is a chemistry teacher at the John D. O’Bryant School of Mathematics and Science, where she has been teaching for 10 years. Mrs. Hibbard has also taught middle school science and marine science, and previously worked as a laboratory technician at the Massachusetts Institute of Technology. She was born in Santiago, Chile where she pursued studies in chemistry and science education. She received her M.Ed. from Cambridge College and holds a Professional Massachusetts Licensure in General Science and Chemistry. Outside of the classroom, Mrs. Hibbard was a leader teacher for the Urban Ecology Institute, Machine Science, and Robolab after school program. She received the 2006 “Robotics Pioneer” and in 2007 was awarded the Urban Ecology Institute “Teacher of the Year” award. She currently resides in Boston, MA with her husband and two daughters.
Mary Kirchhoff
Dr. Mary Kirchhoff is Director of the American Chemical Society Education Division, which serves learners and educators by building communities and providing effective chemistry education products, services, and information. She received her Ph.D. in organic chemistry from the University of New Hampshire and joined the Chemistry Department at Trinity College in Washington, DC, in 1992. Dr. Kirchhoff served as Chair of the Division of Natural Sciences and Mathematics during her tenure at Trinity. She began working in green chemistry as an American Association for the Advancement of Science (AAAS) Environmental Fellow and Visiting Scientist with the U.S. EPA’s green chemistry program. Dr. Kirchhoff joined the American Chemical Society in 2001, serving as Assistant Director of the ACS Green Chemistry Institute for three years before moving to the Education Division. Dr. Kirchhoff was elected an AAAS Fellow in 2006.
Paul Konowicz
Dr. Paul Konowicz is a principal scientist in the Biomaterials and Polymer Chemistry Group at Genzyme Corporation. He earned his Ph.D. in organic chemistry from the University of East Anglia, Norwich UK. He has over 20 years of research and development experience working in the biotechnology and pharmaceutical industries. His interests include the synthesis of small organic molecules and polymers from milligram to kilogram scale and carbohydrate chemistry. The main focus of his work has been on the synthesis of natural and synthetic polymer derivatives for use as medical devices for osteoarthritis and wrinkle correction and products for the sustained release of small molecule or biological therapeutics. He is also involved with multi-disciplinary teams that closely support the development of products through the clinical trial stage to approved medical products. Dr. Konowicz is an avid England cricket fan and also has an appreciation of all things Italian, having lived, worked, and traveled extensively in Italy.
Laurie Langdon
Dr. Laurie Langdon is a research associate in the School of Education at the University of Colorado Boulder. As Co-Director for the Colorado Learning Assistant (LA) program, she works with faculty across eleven STEM departments to effectively use LAs in their courses and also teaches the pedagogy seminar for new LAs. In addition, she co-directs the Noyce Scholarship program. Dr. Langdon has extensive experience working with faculty to implement research-based instruction in General Chemistry courses, both as a post-doctoral fellow at the University of New Hampshire and as a Science Teaching Fellow through the Science Education Initiative at CU. Her current efforts focus on recruiting and preparing future STEM teachers, and chemistry teachers in particular. Dr. Langdon has been a revision team member on three editions of the American Chemical Society’s high school textbook, Chemistry in the Community (ChemCom), and she is a member of ACS’s Advisory Board for their new Chemistry Teacher Education Coalition (CTEC) initiative. Dr. Langdon received her Ph.D. in chemical education from the University of Northern Colorado.
Robert Miller
Dr. Robert Miller is the Vice President of the Biomaterials and Chemistry Division at Genzyme Corporation. He received his B.S. degrees in chemistry and marine chemistry in 1980 from Southampton College of Long Island University and received his Ph.D. in organic chemistry in 1985 from the Rensselaer Polytechnic Institute. For the past 20 years, he has worked on a variety of programs at Genzyme that developed products from the chemical modification of hyaluronan. More recently, he has focused on the development of unique medical devices that will affect the local environment around the implant by delivering drugs or growth factors.
Louisa Morrison
See Louisa Morrison’s biography above.

Daniel Rosenberg
Daniel Rosenberg has primary responsibility for the design and execution of chemistry demonstrations at Harvard University. He is passionate about chemistry and fascinated by physics. As a Science Demonstrator at Harvard, he takes established scientific principles and creates demonstration experiments that make those ideas accessible to everyone. His enthusiastic performance of chemical and physical demonstrations has led to the stage, with “Moments of Science” for the Ig Nobel Prize ceremony in Sanders Theater. He is part of the Harvard Holiday Lecture series, presents an exciting capstone talk with demonstrations for the Harvard Foundation Science Seminar, and does outreach to the Cambridge school community at large. He is a sculptor, inspired by chemical and biological models and ideas, using wire to make kinetic models of RNA and proteins, and mobiles that explore balance and equilibrium.
Wolfgang Rueckner
Dr. Wolfgang Rueckner is the Manager of Lecture Demonstration Services at Harvard University. Dr. Rueckner received his Ph.D. in physics from Brandeis University, with research in high-energy electron impact spectroscopy. Since arriving at Harvard in 1978, his interests have been in science education. Soon after joining the staff at the Harvard Science Center, he formed a lecture-demonstration services group not only to provide for daily class needs but also to continuously improve and develop new demonstration experiments for lectures and instructional laboratories. Dr. Rueckner has also been teaching for the Harvard Extension School since 1995. He has been involved with many science education projects in museums, NOVA films, television shows, and was the resident consulting physicist on NPR’s Car Talk.
Kimberly Stieglitz
Dr. Kimberly Stieglitz is a professor of biotechnology and chemistry at Roxbury Community College and, since 2005, an adjunct professor of chemistry at Bunker Hill Community College. She received her Ph.D. from Boston University Medical School in biophysics and was a visiting professor at the University of Massachusetts Boston (UMB) for two years, teaching biochemistry lecture and laboratory sections in biochemistry and organic chemistry courses. Dr. Stieglitz has published over 23 articles in peer-reviewed journals as well as two laboratory manuals for general chemistry. Her research area of interest is in medical chemistry and structural biology and, in addition to a full-time teaching career, she continues to pursue research collaborations with colleagues from UMB and Boston University. During the summers, she participates in a Research Opportunity for Teachers (RET) program at MIT’s Center for Material Sciences and Engineering (CMSE).
Units
Table of Contents
| Laboratory Safety Atomic Structure Matter Measurement Electrons in Atoms Periodic Table Chemical Bonding Molecular Structure | The Mole Chemical Reactions Stoichiometry Gases Liquids & Solids Solutions Acids & Bases Nuclear Chemistry |
PowerPoint Presentations
Laboratory Safety
Atomic Structure - Ch. 3
Atomic Timeline Song
I. Structure of the Atom
II. Masses of Atoms
Matter - Ch. 1
I. States of Matter
II. Classification of Matter
III. Properties & Changes in Matter
Measurement - Ch. 2
I. Using Measurements
II. Units of Measurement
III. Unit Conversions
Electrons in Atoms - Ch. 4
I. Waves & Particles
II. Bohr Model of the Atom
III. Quantum Model of the Atom
IV. Electron Configuration
Periodic Table - Ch. 6
I. History
II. Organization
III. Periodic Trends
Chemical Bonding - Ch. 6 & 7
Come Together: An Internet Sampler on Chemical Bonding
I. Introduction to Bonding
II. Molecular Compounds
III. Ionic Compounds
IV. Acids
Molecular Structure - Ch. 6
I. Lewis Diagrams
II. Molecular Geometry
III. Molecular Polarity
The Mole - Ch. 3 & 7
I. Molar Conversions
II. Molarity
III. Formula Calculations
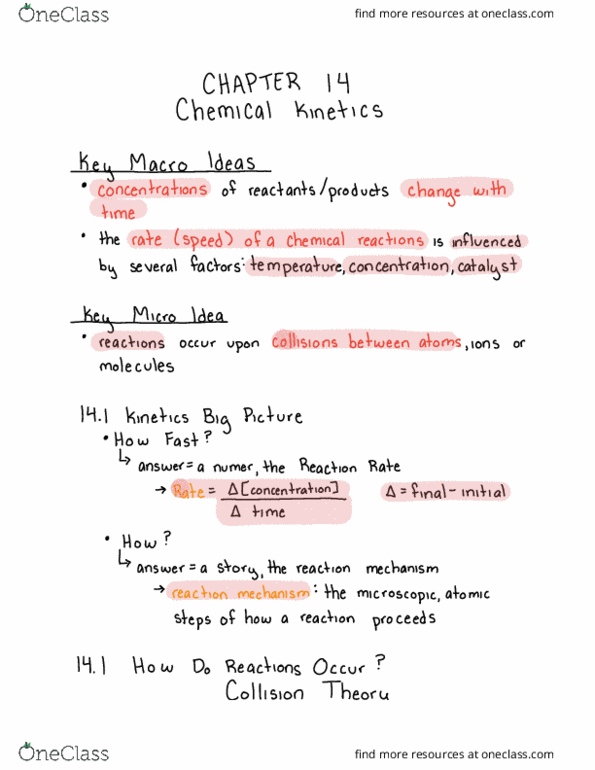
Chemical Reactions - Ch. 8
I. Intro to Reactions
II. Balancing Equations
III. Types of Reactions
IV. Reaction Energy
V. Reaction Rate
Stoichiometry - Ch. 9
I. Stoichiometric Calculations
II. Stoichiometry in the Real World
Chemistry Chapter 9 Chemical Reactions Study Guide Answers
Gases - Ch. 10 & 11
I. Physical Properties of Gases
II. The Gas Laws
III. Ideal Gas Law
IV. Gas Stoichiometry (non-STP)
V. Two More Laws
Gas Laws Practice Problems
Ideal Gas Law & Gas Stoichiometry Practice Problems
Chemistry Chemical Reactions Study Guide Key
Liquids & Solids - Ch. 12
I. Intermolecular Forces
II. Physical Properties
II. Changes of State - Phase Changes, Heating Curves, Phase Diagrams
Solutions - Ch. 13 & 14
I. Nature of Solutions
II. Concentration
III. Colligative Properties
Chemistry Study Guide Unit 3 Chemical Reactions Answer Key
Acids & Bases - Ch. 15 & 16
I. Intro to Acids & Bases
II. pH
III. Titration
Nuclear Chemistry - Ch. 22
I. The Nucleus
II. Radioactive Decay
III. Fission & Fusion
IV. Nuclear Applications



